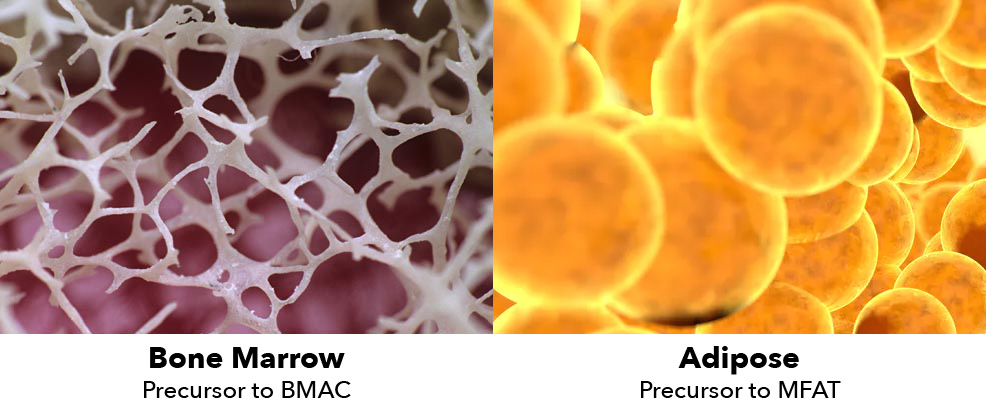
Microfat (MFAT) has many advantages over Bone Marrow Aspirate Concentrate and can usually be tolerated in cases when BMAC cannot.
BMAC and MFAT have a lot in common in orthopedics. They both can provide pain relief in joints, both are autologous, both less invasive and less costly alternatives to surgery, both are orthobiologic, both have very low risk of infection or rejection, both can often be performed in clinic rather than procedure rooms or hospitals, and both have legions of loyal doctor users and satisfied patients. Perhaps you are one of them.
They work differently but both contain mesenchymal stem cells (MSCs) that we believe can restore, revitalize, and regenerate injured or damaged tissue. Since BMAC has been in use longer than MFAT, many doctors who use BMAC haven’t considered MFAT, either because they believe BMAC is superior or they just don’t know enough about MFAT.
Microfat Advantages
Generally, MFAT has some significant advantages over BMAC, including:
- MFAT contains many more MSCs than does BMAC, and MSCs are considered a primary regenerative component to regenerative medicine treatments.
- Stem cells remain numerous in adipose tissue with age while MSCs in BMAC significantly diminish after age 50.
- Some patients feel that removing belly fat, even a small amount, is a very positive bonus. Others feel more comfortable losing fat rather than losing bone marrow (even when aware both the fat and the marrow can be replaced).
- Microfat has cushioning properties that BMAC does not.
- MFAT MSCs can be easily harvested and the procedure is less invasive and less painful than the one performed to obtain BMAC.
- In vitro studies demonstrate that MFAT MSCs maintain their phenotype for a longer period and exhibit higher proliferation rates than BMAC.
- Microfat produced by MiniTC can address up to four joints at the same time for minimal additional expense.
- See https://sheinkopmd.com/power-of-fat-svf-for-orthopaedic-issue/
Additionally, BMAC cannot be used in certain situations, such as if a patient is on a medication that contraindicates BMAC.
When BMAC is not Indicated
Here are some MFAT traits that BMAC-using physicians should consider when there are issues with BMAC:
- About the same cost as BMAC kits.
- May be used for those with systemic diseases such as leukemia and lymphoma after approval by oncologist that disease is in remission.
- May be used on patients using prescription medications that adversely affect bone marrow.
- Affective in Kellgren and Lawrence (K/L) 4 OA.
- May be used within 12 weeks of chemotherapy and irradiation.
- Not affected by most prescription drugs.
- May be used with BMI>32.5.
- For those who have failed BMAC injections.
- Proving to be very effective in Rotator Cuff Pathology.
- May used in almost every setting where BMAC not indicated.
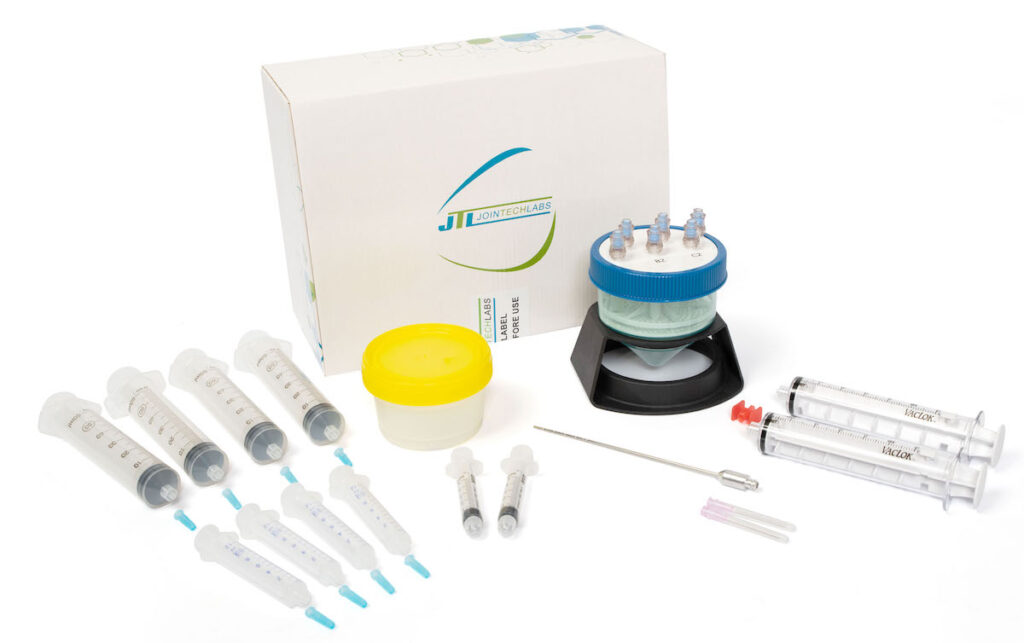
MiniTC Microfat Processing Advantages
MiniTC, the industry-leading microfat processing system from Jointechlabs, has advantages over other MFAT systems, including:
- Highest regenerative cell yield: MiniTC produces the industry’s highest cell yield, leading to superior outcomes.
- Sterile, disposable, all-in-one: Everything comes in a single box and a sterile, disposable process that simplifies procedures.
- Highest quantity of microfat in one use. Can address up to four joints at the same time for minimal additional expense.
- Quick and consistent results: Pure microfat can be produced in just 30 minutes, and the entire procedure takes under an hour. The standardized protocol eliminates manual shaking and produces consistent results unmatched by other solutions.
- Injectable with a fine gauge needle.
- Fits YOUR workflow. MiniTC can be operated by you or your medical staff right in your office or clinic.
- Simple. At the end of the day, MiniTC stands out because it’s fast and easy to use.
- Competitive price: MiniTC is priced lower than other FDA-cleared alternatives.
- FDA cleared and made by Jointechlabs, an ISO 13485:2016 certified company.
- See more at https://Jointechlabs.com/minitc/
Physicians who use BMAC recognize the healing power of orthobiologics. These same physicians owe it to themselves and their patients to consider MFAT and MiniTC.